Clinical Investigator Brochure Template Medical Device
Clinical investigation of medical devices for human subjects good clinical practice.
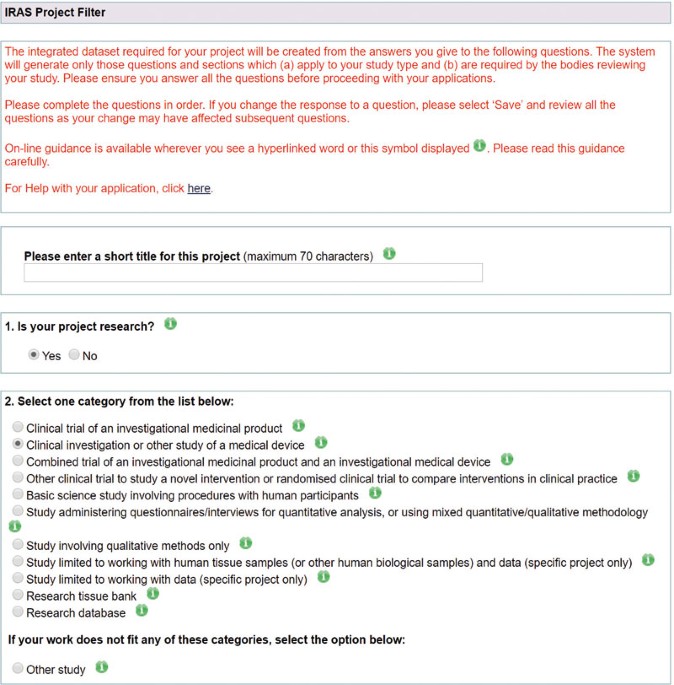
Clinical investigator brochure template medical device. Sample investigator s brochure template free download and preview. 2 1 summary of the literature and clinical evaluation. A summary description of the literature and clinical eva luation and risk analysis that supports.
Investigator s brochure ib a compilation of the clinical and non clinical data on the investigational product s that are relevant to the study of the product s in human subjects. The purpose of the investigator s brochure ib is to provide the principal investigator s with sufficient safety or performance data from pre clinical investigation s and or clinical investigation s to justify human exposure to the investigational device specified in the clinical investigational protocol. Determinant of the investigational medical device safety or performance parameters and is usually used to calculate the sample size.
This 21 page investigator s brochure template is intended to assist you in the process of drafting an investigator s brochure for devices based on ich topic e 6 r1 guideline for good clinical practice. Summary of the medical device and include identification of any features of design that are.