Investigator Brochure Template Ema
The dcsi is an integral part of the investigator s brochure and documents the adverse events which based on the information available so far could be reasonably assumed to be associated with enter compound number and therefore considered expected for the purposes of expedited reporting to regulatory authorities and investigators.
Investigator brochure template ema. Guideline for good clinical practice e6 r2 ema chmp ich 135 1995 page 6 68 introduction good clinical practice gcp is an international ethical and scientific quality standard for designing. This document addresses the good clinical practice an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects it aims to provide a unified standard for the ich regions to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions. The investigator s brochure ib is given to clinicians investigators and other healthcare professionals involved in the conduct of clinical trials for instance the clinical trial coordinators and study nurses.
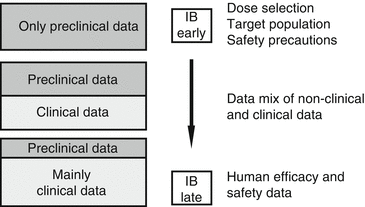
The ib is a compilation of non clinical and clinical data relevant to the study of the medicine in humans it is the single most comprehensive document. Maps investigator s brochure mdma page 5 symptom profiles and to reports from anecdotal research maps is conducting a protocol investigating changes in social anxiety experienced by autistic adults when using two sessions of mdma assisted therapy interspersed with biweekly non drug integration sessions. The table of contents for the ib template is shown in guideline attachment 1.
Investigator s brochure for atmp introduction. This 21 page investigator s brochure template is intended to assist you in the process of drafting an investigator s brochure for devices based on ich topic e 6 r1 guideline for good clinical practice. Update investigator s brochure ib at least once per year according to good clinical practice include any relevant new including safety related data on imp.
Is based on the ema guideline. Investigator s brochure guideline 10 july 2002 4 general format and content of the investigator s brochure the major components and general organization of an ib are given in the ib template and explained further below.