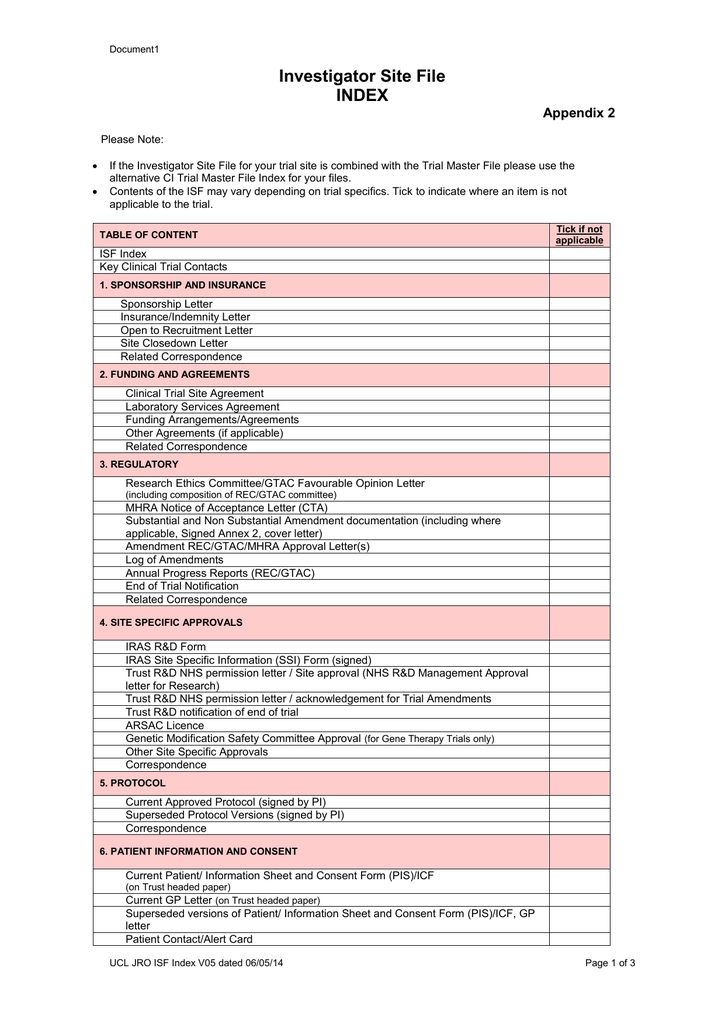
Investigator Brochure Template Mhra
The ib is a compilation of non clinical and clinical data relevant to the study of the medicine in humans it is the single most comprehensive document.
Investigator brochure template mhra. Ucl jro ib template v1 0 14th february 2019 confidential page 4 of 13 1. Medicines and healthcare products regulatory agency mhra uk inspectorate comments on the public consultation of ct3 mhra welcomes the opportunity for review of the proposed document. The investigator s brochure for a non authorised investigational medicinal product.
Investigator s brochure ib ibs are complex documents that provide investigators and ethics committees with a broad overview of a product s development. Investigator s brochure guideline 10 july 2002 4 general format and content of the investigator s brochure the major components and general organization of an ib are given in the ib template and explained further below. The dcsi is an integral part of the investigator s brochure and documents the adverse events which based on the information available so far could be reasonably assumed to be associated with enter compound number and therefore considered expected for the purposes of expedited reporting to regulatory authorities and investigators.
Summary this section should contain a brief maximum of two pages summary highlighting the significant points included in this document. Trilogy has years of experience helping its clients to write concise and well structured ibs. The table of contents for the ib template is shown in guideline attachment 1.
Remember the entire investigators brochure ib is not the rsi but a clearly defined section of it should be if an ib is. As the rsi was not clearly defined there were examples of the same reaction being assessed as expected by one pi and unexpected by another resulting in a large number of susars not being identified or reported to the mhra.