Transcelerate Investigator Brochure Template
Note transcelerate periodically reviews and updates this log if an issued version of the log changes during a study no action is required unless requested by the sponsor.
Transcelerate investigator brochure template. This 21 page investigator s brochure template is intended to assist you in the process of drafting an investigator s brochure for devices based on ich topic e 6 r1 guideline for good clinical practice. Enter name of the sponsor company principal investigator. Scope applicable to all clinical research projects undertaken at melbourne health including investigator initiated research collaborative research and all phases of clinical.
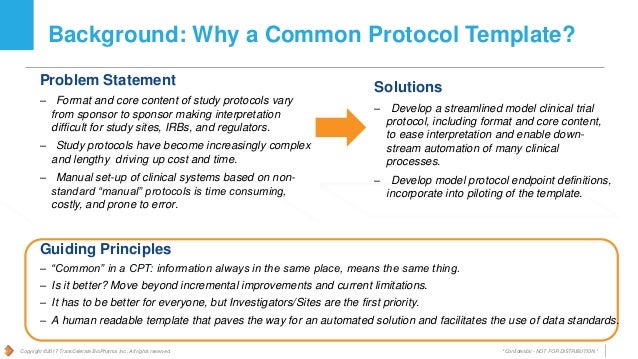
Enter the protocol study. Transcelerate s clinical research access information exchange initiative aims to help the industry better inform patients by facilitating improved touchpoints with information about clinical research and specific clinical trial opportunities. Transcelerate s solutions are designed through collaboration with multiple stakeholders across the ecosystem.
Enter the name of the principal investigator protocol study number. Investigator s brochure guideline 10 july 2002 4 general format and content of the investigator s brochure the major components and general organization of an ib are given in the ib template and explained further below. For additional information please click to our gcp training mutual recognition page.
Is a non profit organization with a mission to collaborate across the global biopharmaceutical research and development community to identify prioritize design and facilitate implementation of solutions designed to drive the efficient effective and high quality delivery of new medicines. Designed with patients for patients. This allows clinical trial investigators and other site personnel to complete gcp training which may be recognized by other transcelerate member companies making it unnecessary to train separately for each participating company.
The table of contents for the ib template is shown in guideline attachment 1.